Water is a polar particle and furthermore goes about as a polar dissolvable. At the point when a synthetic animal category is supposed to be “polar,” it implies that the positive and negative electric charges are unevenly dispersed. The positive charge comes from the nuclear core, while the electrons supply the negative charge. The development of electrons decides the extremity. This is the secret to water.
Learn more here
Why is water a polar particle
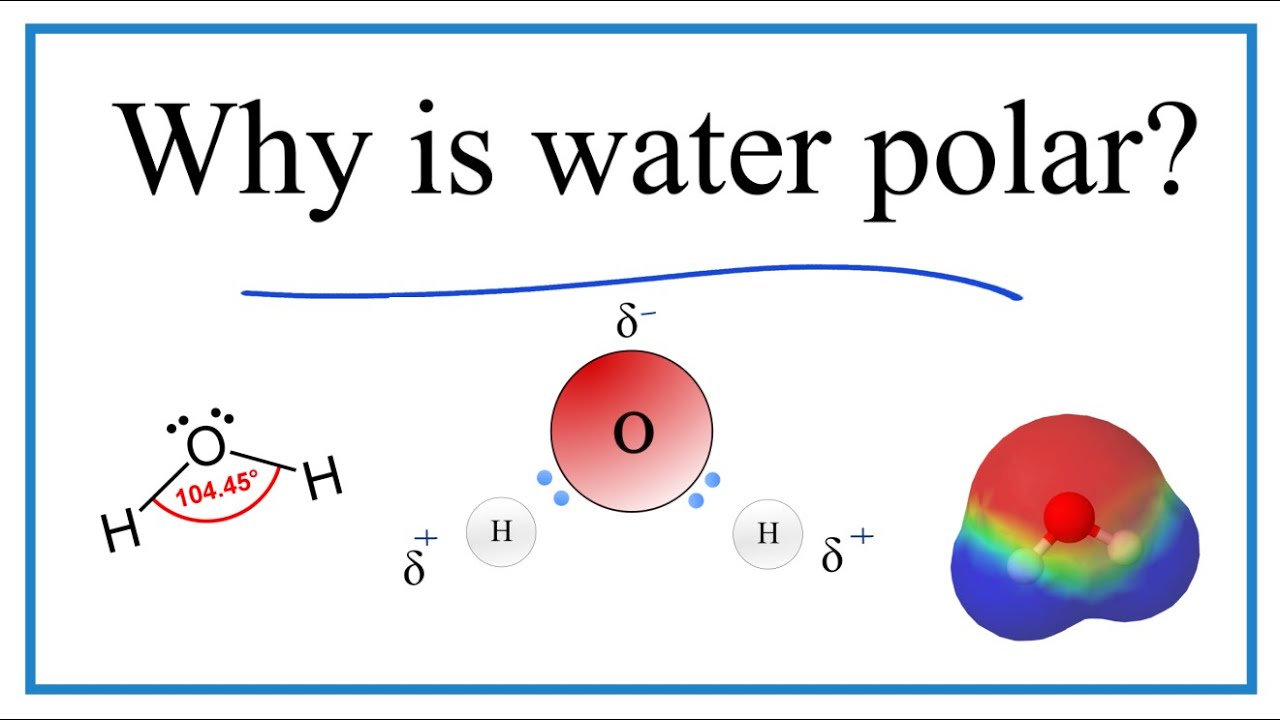
Water is polar in light of the fact that it has a bowed calculation that puts decidedly charged hydrogen iotas on one side of the particle and adversely charged oxygen particles on the opposite side of the atom.
The net impact is a fractional dipole, where the hydrogen molecule has an incomplete positive charge and the oxygen particle has a halfway bad charge.
The explanation water turns is on the grounds that the oxygen particle actually has two solitary sets of electrons, even subsequent to holding with the hydrogen. These electrons repulse one another, bowing the O-H bond away from the direct point.
Learn more about the List of Rick and Morty episodes
The extremity of a water particle
Water (H2O) is polar due to the collapsed state of the atom. The shape implies that the greater part of the negative charge from the oxygen is on one side of the atom and the positive charge of the hydrogen iotas is on the opposite side of the particle. This is an illustration of a polar covalent substance bond. At the point when solutes are added to water, they can be impacted by charge appropriation.
The state of the atom isn’t straight and non-polar (for instance, like CO) because of the distinction in electronegativity between hydrogen and oxygen. The electronegativity worth of hydrogen is 2.1, while that of oxygen is 3.5. The more modest the contrast between the electronegativity esteems, the more iotas will shape a covalent bond. An enormous contrast is seen between electronegativity values with ionic bonds. Hydrogen and oxygen are both going about as nonmetals under ordinary circumstances, however, oxygen is fundamentally more electronegative than hydrogen, so the two particles structure covalent compound security, yet it is polar.
The profoundly electronegative oxygen molecule draws in electrons or a negative charge, making the locale around the oxygen more negative than the district around the two hydrogen particles. The electropositive pieces of the particle (hydrogen molecules) are shifted away from the two filled orbitals of oxygen. Fundamentally, both hydrogen particles are drawn to a similar side of the oxygen iota, however, they are as distant from one another as they can be on the grounds that the hydrogen molecule between the two has a positive charge. The Libra creation is harmony among fascination and repugnance.
Recall that despite the fact that the covalent connection between every hydrogen and oxygen in water is polar, a water particle is an electrically nonpartisan particle by and large. Each water particle has 10 protons and 10 electrons, with a net charge of 0.
Why is water a polar dissolvable
The state of each water atom influences the manner in which it communicates with other water particles and with different substances. Water goes about as a polar dissolvable on the grounds that it tends to be drawn to a positive or negative electric charge on a solute. The marginally bad charge close to the oxygen particle draws in adjacent hydrogen iotas from the decidedly charged locales of water or different atoms. The somewhat sure hydrogen side of each water particle is drawn to other oxygen iotas and adversely charged areas of different atoms. The hydrogen connection between one water atom’s hydrogen and the other’s oxygen keeps water intact and gives it fascinating properties, yet hydrogen bonds are not generally serious areas of strength for so covalent bonds. While water particles are drawn to one another through hydrogen holding, around 20% of them are allowed to collaborate with other synthetic species at some random time. This connection is called hydration or disintegration.